i3- hybridization|sp hybridization chart : Manila Learn how to determine the hybridization of I3-, a linear anion with sp3d hybridization, by using the formula or the lone pairs and valence electrons. See .
SULASOK.TV - The No.1 Best Pinay Sex & Pinay Porn Sites in terms of newest Trending and Viral. Watch free latest and Updated HD videos every day. Watch HD Pinay Porn, Pinay Scandals, Asian Porn, and Western Porn for free. Access a huge database of XXX Adult contents exclusively at SULASOK pornsite.
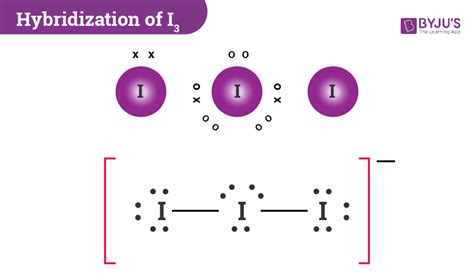
i3- hybridization,Learn how to determine the hybridization of I3-, a linear anion formed by the bonding of I2 with I3. Find out the formula, bond angle, molecular geometry and other important points to remember about I3- hybridization. Tingnan ang higit paTo know the hybridizationof Triiodide ion, we can use simple hybridization formula which is given as; If we look at the iodine atoms there are seven valence electrons in its outer . Tingnan ang higit paI3- molecular geometry is linear. While there are three Iodine atoms, one of the atoms has a negative charge which further gives 3 lone pairs of electrons and 2 bond pairs. Its . Tingnan ang higit pa
Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. . Learn how to draw the Lewis structure of I3- ion, a polyatomic molecule with a negative charge, and how to calculate its hybridization, molecular geometry, and polarity. Find out the properties and uses of I3- . Learn how to determine the hybridization of I3-, a linear anion with sp3d hybridization, by using the formula or the lone pairs and valence electrons. See .
I3- Lewis Structure - Triiodide Ion. This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, .
Hybridisation of a molecule = ( Valence electrons of the central atom + Number of monovalent atoms attached to the central atom + Negative charge on the . The ideal bond angle for the Triiodide Ion is 180° since it has a Linear molecular geometry. Experimentally we would expect the bond angle to be .
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle. How is the structure of triiodide ion (I3-) possible? Ask Question. Asked 5 years, 8 months ago. Modified 1 year, 3 months ago. Viewed 18k times. 13. I have seen the structure of triiodide ion ( IX3X− I .Three atomic orbitals on each carbon – the 2 s, 2 px and 2 py orbitals – combine to form three sp 2 hybrids, leaving the 2 pz orbital unhybridized. The three sp 2 hybrids are .In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3-hybridized, meaning that both have four bonds arranged with tetrahedral geometry.The carbon-carbon bond, with a bond length of 1.54 Å, is formed by overlap of one sp 3 orbital from each of the carbons, while the six .
Triiodide is a model system in photochemistry. Its reaction mechanism has been studied in gas phase, solution and the solid state. In gas phase, the reaction proceeds in multiple pathways that include iodine molecule, metastable ions and iodine radicals as photoproducts, which are formed by two-body and three-body dissociation. This indicates that the hybridization of I3- is sp 3 d. Another way to determine the hybridization of I3- is by counting the number of valence electrons and lone pairs and adding them together. In this case, we have 3 lone pairs and 2 atoms donating valence electrons, giving us a total of 5, which also indicates sp 3 d hybridization. Key .Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon's 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is . I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle. Orbital hybridization The observation of molecules in the various electronic shapes shown above is, at first blush, in conflict with our picture of atomic orbitals. For an atom such as oxygen, we know that the 2s orbital is spherical, and that the 2p x , 2p y , and 2p z orbitals are dumbell-shaped and point along the Cartesian axes.i3- hybridization sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of .
HYBRIDIZATION OF I3. To know the crossbreeding of Triiodide particle, we will use a straightforward crossbreeding formula that is given as; Number of crossbreeding = electron + monovalent + (negative charge) – (positive charge)/2. If we glance at the iodine atoms, there are seven valence electrons in its outer shell, and 2 monovalent atoms .
Triiodide [I3]- ion Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, hybridization, formal charges, polar vs non-polar. The chemical formula I 3– represents an anion composed of three iodine (I) atoms. This anion is known as the triiodide ion. The triiodide (I 3–) ion is generated by the chemical reaction of .

This video illustrates a step-by-step process in deriving the electronic geometry, molecular geometry, hybridization of the central atom, and formal charge o.sp Hybridization. The beryllium atom in a gaseous BeCl 2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms. There are two regions of valence electron .Solution. The correct option is D Both are planar species. Here the hybridisation of central atom in I + 3 is sp3 and it contains 2 lone pairs on it. So, I + 3 becomes bent shaped, planar and is polar. The hybridisation of central atom in I − 3 is sp3d and it contains 3 lone pairs on it. The lone pairs are present on equatorial axis and two .
Hybridization of s and p Orbitals. In BeH 2, we can generate two equivalent orbitals by combining the 2s orbital of beryllium and any one of the three degenerate 2p orbitals. By taking the sum and the difference .Click here:point_up_2:to get an answer to your question :writing_hand:the hybridisation of central iodine atom in if5 i3 and i3 are respectivelyi3- hybridization sp hybridization chart Prediction of sp 3 d, sp 3 d 2, and sp 3 d 3 Hybridization States. In case of sp 3 d, sp 3 d 2 and sp 3 d 3 hybridization state there is a common term sp 3 for which 4 sigma bonds are responsible. So, in addition to 4 sigma bonds, for each additional sigma, added one d orbital gradually as follows:-5σ bonds = 4σ bonds + 1 additional σ bond = .Answer (c): Hybridization using d orbitals allows chemists to explain the structures and properties of many molecules and ions. Like most such models, however, it is not universally accepted. Nonetheless, it does explain a fundamental difference between the chemistry of the elements in the period 2 (C, N, and O) and those in period 3 and below .
sp hybridization chart I3- ion has sp3d hybridization and linear shape containing two bond pair and three lone pair while I3+ ion has sp3 hybridization and bent shape containing two bond pair and two lone pair. Related Questions: Why aqueous solution of AlCl3 is acidic in nature ? What happen when aq AlCl3 react with Acid or Base?
I3- is sp3d hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the Iodine. The VSEPR predicts the linear shape. Three of the sp3d orbitals are occupied by 3 lone pairs and the other two orbitals form bonds with the other Iodine atoms.'. Suggest Corrections. 1.
i3- hybridization|sp hybridization chart
PH0 · what is hybridization in chemistry
PH1 · what does sp3 hybridized mean
PH2 · sp hybridization chart
PH3 · lone pairs in i3
PH4 · i3 lewis structure molecular geometry
PH5 · i3 hybridization of central atom
PH6 · i3 electron domain geometry
PH7 · hybridization chemistry for dummies
PH8 · Iba pa